- Who was available from the study to assist us with questions as they come up for the protocol?
- How long can preservation time be per stage?
- How long does the EVLP process take from initiation to completion?
- Did study centers receive an alert when a lung is sent for EVLP?
- Was there any reference that needs to be followed for procuring the lungs for this study?
- Who was responsible for transportation arrangements to and from LBE facilities?
- Can a single lung be perfused on your system?
- Was ECMO an exclusion criterion?
- If a patient previously had a right single transplant and then needs a transplant on the left, were they eligible for the study?
- What was the definition of PGD for double lungs and single lungs?
- What type of training do the Specialists have?
Who was available from the study to assist us with questions as they come up for the protocol?
Lung Bioengineering worked with a clinical research organization (CRO), a resource for questions related to data collection and/or analysis and adverse events. In general, study centers would first consult with the CRO when questions about the protocol arise.
The Lung Bioengineering team was available to consult with study center physicians/surgeons about suitability criteria for lungs undergoing EVLP or post-EVLP suitability for transplantation.
How long can preservation time be per stage?
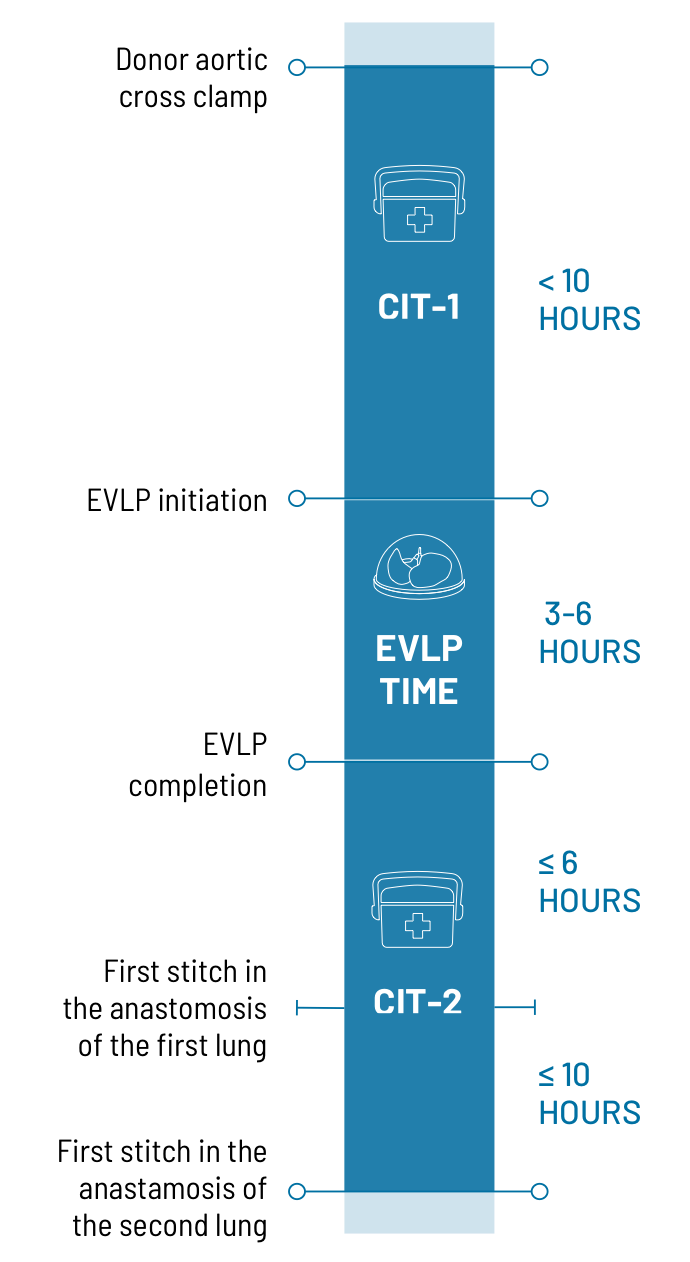
CIT-1, from donor aortic cross clamp to the start of the EVLP process at LBE facilities, must be 10 hours or less.
EVLP time, from initiation to completion of EVLP procedure may be a minimum of 3 hours and a maximum of 6 hours.
CIT-2, from completion of EVLP to the first stitch in the anastomosis of the first lung, must be less than or equal to 6 hours. For sequential bilateral procedures, CIT-2 for the second lung must be less than or equal to 10 hours.
How long does the EVLP process take from initiation to completion?
EVLP begins once lung perfusion starts on the EVLP circuit. The EVLP procedure runs for a minimum of 3 hours up to a maximum of 6 hours.

Did the study centers receive an alert when lungs were sent for EVLP?
The involved transplant program received electronic alerts when a lung arrived at LBE facilities. At the conclusion of the EVLP procedure, the study center received another alert telling the transplant physician to log in and entered their disposition of the lung (accept or decline for transplant).
Was there any reference that needs to be followed for procuring the lungs for this study?
The protocol had no requirements for the way that the lungs are procured. However, we did have a “Lung Retrieval and Preservation for EVLP” reference that has recommendations for lung retrieval specifically for EVLP.
Who was responsible for transportation arrangements to and from LBE facilities?
The transplant center and/or OPO were in charge of arranging air transportation, and LBE was responsible for local ground transportation to the EVLP facility. If, for any reason, transportation arrangements could not be made expeditiously, Lung Bioengineering was able to assist with air and ground transportation logistics. An EVLP Specialist contacted the appropriate transplant center personnel to ensure that lungs were imported and exported as quickly as possible.
Can a single lung be perfused on your system?
Yes. A single lung can be perfused on the Centralized Lung Evaluation System.
Was ECMO an exclusion criterion?
No, ECMO was not an exclusionary criterion. Pre-transplant ECMO status was tracked for all enrolled subjects as Non-EVLP group subjects were matched on several criteria, including ECMO.
If a patient previously had a right single transplant and then needs a transplant on the left, were they eligible for the study?
Yes, they were eligible for the study because it is NOT a same side re-transplant.
What was the definition of PGD for double lungs and single lungs?
Primary Graft Dysfunction (PGD) Score
The subject’s PGD Score was determined upon admission to the ICU after transplantation, as well as 24, 48, and 72 hrs post-transplant following the 2016 ISHLT PGD definition.1 PGD Score was graded using the parameters in Table 2, where the P/F ratio is PaO2/FiO2.
Table 2 | Primary Graft Dysfunction Grading
| Grade | P/F Ratio | Pulmonary Edema on Chest X-ray |
|---|---|---|
| 0 | ANY | No |
| 1 | >300 | Yes |
| 2 | 200 TO 300 | Yes |
| 3 | <200 | Yes |
Grade severity notes: Patients with no evidence of pulmonary edema on chest X-ray were considered grade 0. Absence of invasive mechanical ventilation was graded according to the PaO2/FIO2 ratio, using methods similar to those used for patients receiving mechanical ventilation. If PaO2 was not available for calculation of a PaO2/FIO2 ratio, then an oxygen saturation/FIO2 ratio was calculated, and the 200 and 300 PGD grading cutoffs were adjusted to 235 and 315. Use of nitric oxide, aerosolized epoprostenol, or other pharmacologic agents that may have improved oxygenation did not change grading methods. Use of extracorporeal lung support (ECLS) with bilateral pulmonary edema on chest X-ray imaging was graded as grade 3, and ECLS use was explicitly recorded. The use of ECLS for non-hypoxic indications without pulmonary edema on chest X-ray imaging was considered ungradable and explicitly recorded separately.
Time window notes: PGD was graded at 4 time points over the first 72 hours after transplantation (T0, T24, T48, and T72 hours). T0 was defined as the time of the first blood draw for blood gas testing after admission to the ICU, which took place within 6 hours of reperfusion of the last lung transplanted. T24, T48, and T72 had time windows of ±6 hours. If multiple blood gas values were available, the worst PaO2/FIO2 ratio in a given assessment was used.
Other notes: PaO2/FIO2 was ideally measured on a positive end-expiratory pressure of 5 cm H2O at an FIO2 of 1.0 while patients were on mechanical ventilation; however, grading was not altered for other settings.
1. Snell GI, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017 Oct;36(10):1097-1103.
What type of training do the Specialists have?
EVLP Specialists candidates are selected from applicants having organ procurement and evaluation experience, including OPO personnel and surgeons. Specialist candidates are then qualified by a combination of intensive training with the development team at Toronto General Hospital, and a Lung Bioengineering-specific competency and qualification program upon their return.
Specialist candidates have extensive training through an in-residence program at TGH, culminating in successful completion of an Ex Vivo Lung Specialist Final Exam. This training program was developed by the team that developed the Toronto EVLP Method, and is therefore specifically focused on this mode of EVLP.
The practical, hands-on experience includes observing and participating in ongoing research (animal) EVLP procedures. Additionally, the specialist candidate will observe and participate in clinical (human) EVLP procedures performed at Toronto General Hospital. This includes assembly of the EVLP components, troubleshooting all equipment, cannulation and intubation of lungs, maintaining a lung on EVLP, explaining donor lung physiology and suitability for EVLP, and interpreting key assessment parameters during the EVLP procedure.